Characteristics of Chemical reactions
- The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be easily observed easily.
- The important characteristics of chemical reactions are:
- Evolution of a gas
- Formation of a precipitate
- Change in colour
- Change in temperature
- Change in state
Balanced and unbalanced chemical equations:
A balanced chemical equation has an equal number of atoms of different elements in the reactants and products.Zinc metal reacts with dilute sulphuric acid to form zinc sulphate and hydrogen gas.
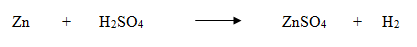
Count the number of atoms of all the elements in the reactants and products separately.
| In reactants | In products | |
| No. of Zn atoms | 1 | 1 |
| No. of H atoms | 2 | 2 |
| No. of S atoms | 1 | 1 |
| No. of O atoms | 4 | 4 |
- There are an equal number of atoms of different elements in the reactants and products, so the above chemical equation is a balanced equation.
- An unbalanced chemical equation has an unequal number of atoms of one or more elements in the reactants and products.
- Hydrogen reacts with oxygen to form water
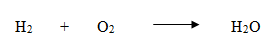
Count the number of atoms of all the elements in the reactants and products separately.
| In reactants | In products | |
| No. of H atoms | 2 | 2 |
| No. of O atoms | 2 | 1 |
Types of chemical reactions
Some of the important types of chemical reactions are:- Combination reactions
- Decomposition reaction
- Displacement reactions
- Double displacement reactions
- Oxidation and Reduction reactions
Combination reactions:
Those reactions, in which two or more substances combine to form a single substance, are called combination reaction.- In a combination reaction, two or more elements can combine to form a compound; two or more compounds can combine to form a new compound; or an element and a compound can combine to form a new compound. Examples:
Hydrogen burns in oxygen to form water: 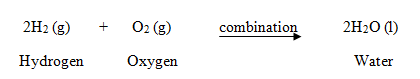 In this reaction, two elements, hydrogen and oxygen are combining to form single compound water, so this is an example of a combination.
In this reaction, two elements, hydrogen and oxygen are combining to form single compound water, so this is an example of a combination.- Ammonia reacts with hydrogen chloride to form ammonium chloride.

Decomposition reactions:
- Those reactions in which a compound splits up into two or more simpler substances are known as decomposition reactions.
The decomposition reactions are carried out by applying heat, light or electricity.
Example: When calcium carbonate is heated, it decomposes to give calcium oxide and carbon dioxide: 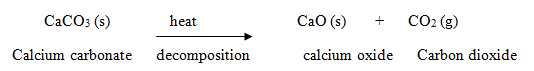 In this reaction, one substance, calcium carbonate is breaking up into two simpler substances, calcium oxide and carbon dioxide, so this is a decomposition reaction.
In this reaction, one substance, calcium carbonate is breaking up into two simpler substances, calcium oxide and carbon dioxide, so this is a decomposition reaction.
- When a decomposition reaction is carried out by heating, it is called ‘thermal decomposition’.
Example: When lead nitrate is heated strongly, it breaks down to form simpler substances like lead monoxide, nitrogen oxide and oxygen.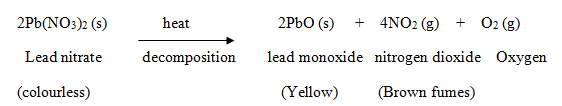
- Some decomposition reactions are carried out by using electricity.
Example: When electric current is passed through acidified water, it decomposes to give hydrogen gas and oxygen gas.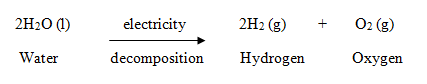 This decomposition reaction takes place by the action of electricity. It is called electrolysis of water.
This decomposition reaction takes place by the action of electricity. It is called electrolysis of water.
- Some decomposition reactions are carried out by light energy.
Example: When silver chloride is exposed to light, it decomposes to form silver metal and chlorine gas.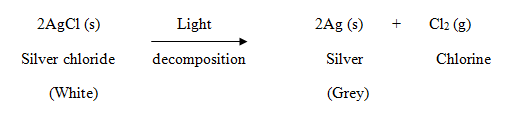
No comments:
Post a Comment